Implanting Hope in the Brain
A new generation of clinical trials is testing whether lab-grown dopamine-producing cells can be implanted directly into the brains of Parkinson’s disease patients to restore lost motor function. The approach, long considered one of the most promising frontiers in regenerative medicine, is now advancing through early-stage human trials at multiple sites across the United States.
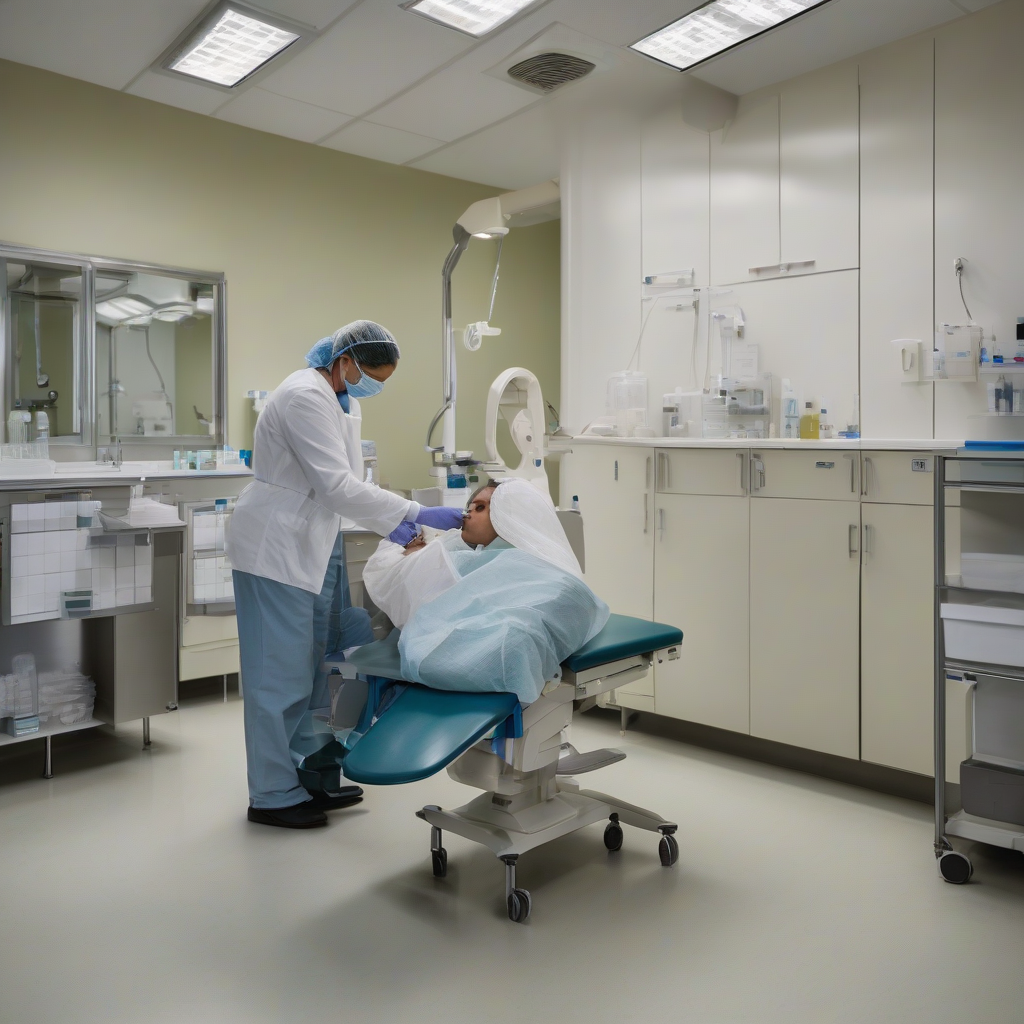
At Keck Medicine of USC, one of three participating U.S. institutions, neurosurgeons are implanting stem cell-derived neurons into the basal ganglia of patients with moderate to moderate-severe Parkinson’s disease. The procedure involves creating a small opening in the skull and using MRI guidance to precisely deliver the cells to the brain region most affected by the disease, as reported by ScienceDaily.
How the Treatment Works
Parkinson’s disease is driven by the progressive loss of neurons that produce dopamine, a chemical messenger essential for coordinating movement, memory, and mood. As these cells die off, patients develop the hallmark symptoms of the disease: tremors, muscle rigidity, slowed movement, and difficulty with balance.
The therapy being tested, known as RNDP-001 and produced by Kenai Therapeutics, uses induced pluripotent stem cells — adult cells taken from skin or blood that have been reprogrammed back to a flexible, embryonic-like state. These reprogrammed cells are then coaxed into becoming dopamine-producing neurons before being transplanted into the patient’s brain.
The goal is for these implanted cells to integrate into existing neural circuits and begin producing dopamine naturally, effectively replacing the neurons that the disease has destroyed. If successful, the approach could offer something that current medications cannot: a way to address the root cause of Parkinson’s rather than merely managing its symptoms.
The Clinical Trial
The Phase 1 REPLACE trial has enrolled 12 participants and has received fast-track designation from the U.S. Food and Drug Administration, reflecting the agency’s recognition of the therapy’s potential to address a serious unmet medical need. Patients undergo close observation for 12 to 15 months following the procedure, with extended follow-up continuing for up to five years to assess long-term safety and outcomes.
As an early-stage trial, the primary focus is on establishing the safety of the procedure rather than measuring efficacy. However, researchers will also be tracking motor function and other clinical measures that could provide early signals of therapeutic benefit.
A Decades-Long Quest
The concept of replacing lost dopamine neurons through transplantation has been pursued since the 1980s, when researchers first attempted to graft fetal brain tissue into Parkinson’s patients. Those early efforts produced mixed results and raised significant ethical concerns, but they established the scientific rationale that has guided the field ever since.
The advent of induced pluripotent stem cell technology has transformed the landscape, offering a renewable and ethically less contentious source of transplantable cells. Several parallel efforts are now underway globally, including trials using human embryonic stem cells and other cell-based approaches.
What Comes Next
Researchers caution that even the most promising early results would need to be replicated in larger, controlled trials before any stem cell therapy could become a standard treatment for Parkinson’s. The disease affects an estimated one million Americans and more than ten million people worldwide, with numbers expected to rise as the global population ages.
For now, the ongoing trials represent a critical proof of concept. If the implanted cells prove safe and show signs of restoring dopamine production, they could open the door to a fundamentally new approach to treating not just Parkinson’s but potentially other neurodegenerative conditions as well.


Reader Comments